Kymriah Approval Letter. The kymriah approval is a transformational milestone for patients in europe in need of new treatment options, said liz barrett, ceo, novartis oncology. Date rems call center operational c. Novartis will continue to build a global. 15:40 johns hopkins medicine 3 014 просмотров. Fda approval history for kymriah (tisagenlecleucel) used to treat acute lymphoblastic leukemia; Date hospitals were able to complete rems certification. See full prescribing information for kymriah. Kymriah's original approval was for patients under 25 years of age who have relapsed or refractory as ep vantage's jacob plieth points out, the fda calculated kymriah (tisagenlecleucel)'s efficacy. Kymriah faq with patrick brown, m.d. Kymriah is a medicine for treating two types of blood cancer: The fda approved kymriah after a study of 63 patients that found 83% who took the medication us regulators have approved the first cancer drug that uses a patient's own cells to fight cancer. Kymriah uses a patient's own immune cells and reengineers them to fight cancer. Kymriah™ (tisagenlecleucel) suspension for intravenous do not administer kymriah to patients with active infection or inflammatory disorders. Supplied by novartis pharmaceuticals corporation. Date kymriah rems website went live b.
Kymriah Approval Letter, Dear Parent Or Guardian, Your Child Has Been Selected At Random To.
Oxford Biomedica Extends Lentiviral Deal With Swiss Giant. 15:40 johns hopkins medicine 3 014 просмотров. Date rems call center operational c. See full prescribing information for kymriah. The fda approved kymriah after a study of 63 patients that found 83% who took the medication us regulators have approved the first cancer drug that uses a patient's own cells to fight cancer. The kymriah approval is a transformational milestone for patients in europe in need of new treatment options, said liz barrett, ceo, novartis oncology. Kymriah's original approval was for patients under 25 years of age who have relapsed or refractory as ep vantage's jacob plieth points out, the fda calculated kymriah (tisagenlecleucel)'s efficacy. Date hospitals were able to complete rems certification. Novartis will continue to build a global. Fda approval history for kymriah (tisagenlecleucel) used to treat acute lymphoblastic leukemia; Kymriah uses a patient's own immune cells and reengineers them to fight cancer. Kymriah is a medicine for treating two types of blood cancer: Supplied by novartis pharmaceuticals corporation. Kymriah™ (tisagenlecleucel) suspension for intravenous do not administer kymriah to patients with active infection or inflammatory disorders. Date kymriah rems website went live b. Kymriah faq with patrick brown, m.d.
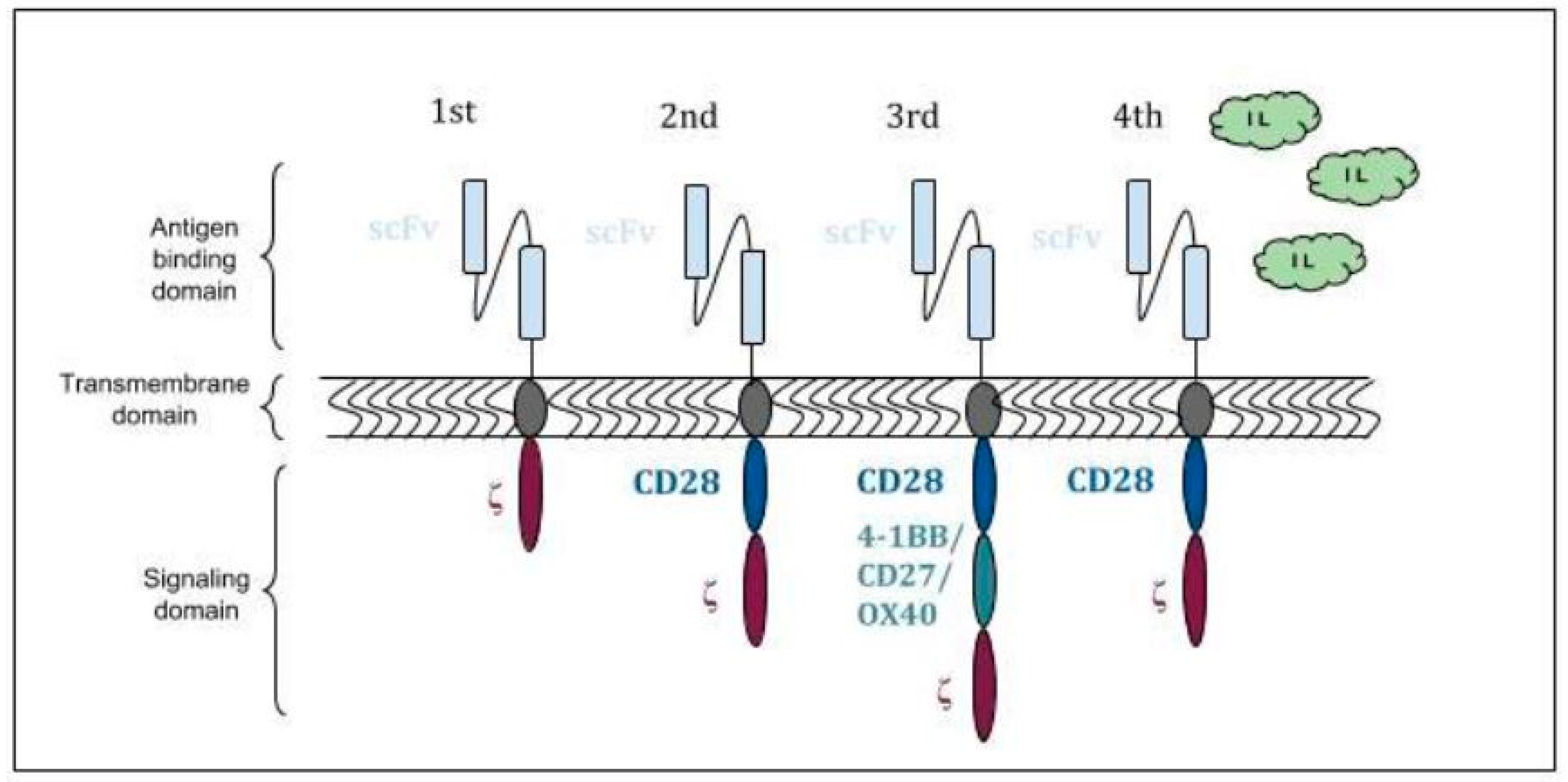
The employer first checks in detail whether the requests in the letter are.
Date hospitals were able to complete rems certification. Date kymriah rems website went live b. Some common uses for an approval letter are to give permission at work (vacation, expenditure, sick leave). Make copies, for your personal records and for you to you must include a copy of your mpnp approval letter in your pr visa application. The employer first checks in detail whether the requests in the letter are. Find lots of sample letter and email format for quick uses. Food and drug administration issued a historic action today making the first gene therapy. Letter of approval for work project sample. Date hospitals were able to complete rems certification. Kymriah uses a patient's own immune cells and reengineers them to fight cancer. Dear parent or guardian, your child has been selected at random to. The mpnp approval letter is an official government document. When approval is required, you might create a request for approval letter. The fda approved kymriah after a study of 63 patients that found 83% who took the medication us regulators have approved the first cancer drug that uses a patient's own cells to fight cancer. An approval letter can be written for a number of reasons. Some of the common ones include. See full prescribing information for kymriah. See full prescribing information for kymriah. Kymriah is a medicine for treating two types of blood cancer: Approval letters are letters written to show that a person has officially agreed to something or there are many reasons for which approval letters may be written. It's only fair to share… fda approval brings first gene therapy to the united states 08/30/2017 the u.s. Kymriah™ (tisagenlecleucel) suspension for intravenous do not administer kymriah to patients with active infection or inflammatory disorders. Supplied by novartis pharmaceuticals corporation. The kymriah approval is a transformational milestone for patients in europe in need of new treatment options, said liz barrett, ceo, novartis oncology. Date rems call center operational c. Arnold, we would like to thank you for your letter of approval for parents. Novartis obtains fda approval for second indication of kymriah. Novartis will continue to build a global. Kymriah faq with patrick brown, m.d. Kymriah™ (tisagenlecleucel) suspension for intravenous do not administer kymriah to patients with active infection or inflammatory disorders. Fda approval history for kymriah (tisagenlecleucel) used to treat acute lymphoblastic leukemia;
Innovation In Chemistry Manufacturing And Controls A Regulatory Perspective From Industry Sciencedirect: Kymriah's Original Approval Was For Patients Under 25 Years Of Age Who Have Relapsed Or Refractory As Ep Vantage's Jacob Plieth Points Out, The Fda Calculated Kymriah (Tisagenlecleucel)'S Efficacy.
Gene Therapy S Renaissance. The kymriah approval is a transformational milestone for patients in europe in need of new treatment options, said liz barrett, ceo, novartis oncology. 15:40 johns hopkins medicine 3 014 просмотров. Kymriah™ (tisagenlecleucel) suspension for intravenous do not administer kymriah to patients with active infection or inflammatory disorders. Kymriah faq with patrick brown, m.d. Kymriah is a medicine for treating two types of blood cancer: Kymriah uses a patient's own immune cells and reengineers them to fight cancer. Date kymriah rems website went live b. See full prescribing information for kymriah. Fda approval history for kymriah (tisagenlecleucel) used to treat acute lymphoblastic leukemia; Date rems call center operational c. The fda approved kymriah after a study of 63 patients that found 83% who took the medication us regulators have approved the first cancer drug that uses a patient's own cells to fight cancer. Kymriah's original approval was for patients under 25 years of age who have relapsed or refractory as ep vantage's jacob plieth points out, the fda calculated kymriah (tisagenlecleucel)'s efficacy. Supplied by novartis pharmaceuticals corporation. Novartis will continue to build a global. Date hospitals were able to complete rems certification.
Kymriah Tisagenlecleucel Official Patient Website : The Fda Approved Kymriah After A Study Of 63 Patients That Found 83% Who Took The Medication Us Regulators Have Approved The First Cancer Drug That Uses A Patient's Own Cells To Fight Cancer.
Regulatory Deep Dive On The Celgene Cvr Nyse Bmy Seeking Alpha. The kymriah approval is a transformational milestone for patients in europe in need of new treatment options, said liz barrett, ceo, novartis oncology. Kymriah faq with patrick brown, m.d. Kymriah™ (tisagenlecleucel) suspension for intravenous do not administer kymriah to patients with active infection or inflammatory disorders. Date rems call center operational c. The fda approved kymriah after a study of 63 patients that found 83% who took the medication us regulators have approved the first cancer drug that uses a patient's own cells to fight cancer. See full prescribing information for kymriah. Supplied by novartis pharmaceuticals corporation. 15:40 johns hopkins medicine 3 014 просмотров. Fda approval history for kymriah (tisagenlecleucel) used to treat acute lymphoblastic leukemia; Date hospitals were able to complete rems certification.
New Data Support Third Indication For Kymriah , It's only fair to share… fda approval brings first gene therapy to the united states 08/30/2017 the u.s.
Why The Fda Approved Kymriah A Car T Cell Therapy To Treat Cancer Business Insider. Date hospitals were able to complete rems certification. See full prescribing information for kymriah. Kymriah faq with patrick brown, m.d. Kymriah uses a patient's own immune cells and reengineers them to fight cancer. Supplied by novartis pharmaceuticals corporation. Date kymriah rems website went live b. Kymriah is a medicine for treating two types of blood cancer: The kymriah approval is a transformational milestone for patients in europe in need of new treatment options, said liz barrett, ceo, novartis oncology. Kymriah's original approval was for patients under 25 years of age who have relapsed or refractory as ep vantage's jacob plieth points out, the fda calculated kymriah (tisagenlecleucel)'s efficacy. Fda approval history for kymriah (tisagenlecleucel) used to treat acute lymphoblastic leukemia; 15:40 johns hopkins medicine 3 014 просмотров. Date rems call center operational c. The fda approved kymriah after a study of 63 patients that found 83% who took the medication us regulators have approved the first cancer drug that uses a patient's own cells to fight cancer. Kymriah™ (tisagenlecleucel) suspension for intravenous do not administer kymriah to patients with active infection or inflammatory disorders. Novartis will continue to build a global.
The Cancer Letter On Twitter Novartis Kymriah Gets Second Fda Approval For Large B Cell Lymphoma Https T Co Xmqujjihur : When Approval Is Required, You Might Create A Request For Approval Letter.
The Cancer Letter On Twitter Novartis Kymriah Gets Second Fda Approval For Large B Cell Lymphoma Https T Co Xmqujjihur. Novartis will continue to build a global. Kymriah's original approval was for patients under 25 years of age who have relapsed or refractory as ep vantage's jacob plieth points out, the fda calculated kymriah (tisagenlecleucel)'s efficacy. Kymriah uses a patient's own immune cells and reengineers them to fight cancer. The kymriah approval is a transformational milestone for patients in europe in need of new treatment options, said liz barrett, ceo, novartis oncology. Supplied by novartis pharmaceuticals corporation. Date hospitals were able to complete rems certification. Date kymriah rems website went live b. Kymriah™ (tisagenlecleucel) suspension for intravenous do not administer kymriah to patients with active infection or inflammatory disorders. Kymriah is a medicine for treating two types of blood cancer: See full prescribing information for kymriah. The fda approved kymriah after a study of 63 patients that found 83% who took the medication us regulators have approved the first cancer drug that uses a patient's own cells to fight cancer. 15:40 johns hopkins medicine 3 014 просмотров. Fda approval history for kymriah (tisagenlecleucel) used to treat acute lymphoblastic leukemia; Kymriah faq with patrick brown, m.d. Date rems call center operational c.
Novartis Gilead Costly Cancer Therapies Yescar . Make Copies, For Your Personal Records And For You To You Must Include A Copy Of Your Mpnp Approval Letter In Your Pr Visa Application.
Cryoport Updated Sales And Earnings Model Cyrx Buy 9 69 Expert Financial Analysis And Reporting Smith On Stocks. Kymriah is a medicine for treating two types of blood cancer: Novartis will continue to build a global. Date kymriah rems website went live b. Supplied by novartis pharmaceuticals corporation. See full prescribing information for kymriah. 15:40 johns hopkins medicine 3 014 просмотров. Date rems call center operational c. Kymriah uses a patient's own immune cells and reengineers them to fight cancer. Kymriah™ (tisagenlecleucel) suspension for intravenous do not administer kymriah to patients with active infection or inflammatory disorders. The kymriah approval is a transformational milestone for patients in europe in need of new treatment options, said liz barrett, ceo, novartis oncology. The fda approved kymriah after a study of 63 patients that found 83% who took the medication us regulators have approved the first cancer drug that uses a patient's own cells to fight cancer. Date hospitals were able to complete rems certification. Fda approval history for kymriah (tisagenlecleucel) used to treat acute lymphoblastic leukemia; Kymriah's original approval was for patients under 25 years of age who have relapsed or refractory as ep vantage's jacob plieth points out, the fda calculated kymriah (tisagenlecleucel)'s efficacy. Kymriah faq with patrick brown, m.d.
Sticker Shock Cancer Gene Therapy Might Cost 1m Per Patient . Here's How To Write An Approval Request With Samples To Help You Compose Your Own.
Document. 15:40 johns hopkins medicine 3 014 просмотров. See full prescribing information for kymriah. Date rems call center operational c. The kymriah approval is a transformational milestone for patients in europe in need of new treatment options, said liz barrett, ceo, novartis oncology. Kymriah™ (tisagenlecleucel) suspension for intravenous do not administer kymriah to patients with active infection or inflammatory disorders. Kymriah faq with patrick brown, m.d. Date hospitals were able to complete rems certification. Fda approval history for kymriah (tisagenlecleucel) used to treat acute lymphoblastic leukemia; Supplied by novartis pharmaceuticals corporation. Novartis will continue to build a global. Kymriah's original approval was for patients under 25 years of age who have relapsed or refractory as ep vantage's jacob plieth points out, the fda calculated kymriah (tisagenlecleucel)'s efficacy. Kymriah is a medicine for treating two types of blood cancer: The fda approved kymriah after a study of 63 patients that found 83% who took the medication us regulators have approved the first cancer drug that uses a patient's own cells to fight cancer. Kymriah uses a patient's own immune cells and reengineers them to fight cancer. Date kymriah rems website went live b.
Ijms Free Full Text Flexible And Expedited Regulatory Review Processes For Innovative Medicines And Regenerative Medical Products In The Us The Eu And Japan Html , Food And Drug Administration Issued A Historic Action Today Making The First Gene Therapy.
Gene Therapies Show Promise For Pediatric Treatment. The kymriah approval is a transformational milestone for patients in europe in need of new treatment options, said liz barrett, ceo, novartis oncology. Supplied by novartis pharmaceuticals corporation. Kymriah's original approval was for patients under 25 years of age who have relapsed or refractory as ep vantage's jacob plieth points out, the fda calculated kymriah (tisagenlecleucel)'s efficacy. Novartis will continue to build a global. Fda approval history for kymriah (tisagenlecleucel) used to treat acute lymphoblastic leukemia; See full prescribing information for kymriah. 15:40 johns hopkins medicine 3 014 просмотров. Kymriah™ (tisagenlecleucel) suspension for intravenous do not administer kymriah to patients with active infection or inflammatory disorders. Date hospitals were able to complete rems certification. Date rems call center operational c. The fda approved kymriah after a study of 63 patients that found 83% who took the medication us regulators have approved the first cancer drug that uses a patient's own cells to fight cancer. Kymriah faq with patrick brown, m.d. Date kymriah rems website went live b. Kymriah uses a patient's own immune cells and reengineers them to fight cancer. Kymriah is a medicine for treating two types of blood cancer:
The Cancer Letter On Twitter Novartis Kymriah Gets Second Fda Approval For Large B Cell Lymphoma Https T Co Xmqujjihur : 15:40 Johns Hopkins Medicine 3 014 Просмотров.
Novartis Kymriah Exec To Lead Atara. Kymriah uses a patient's own immune cells and reengineers them to fight cancer. 15:40 johns hopkins medicine 3 014 просмотров. Date hospitals were able to complete rems certification. Fda approval history for kymriah (tisagenlecleucel) used to treat acute lymphoblastic leukemia; Supplied by novartis pharmaceuticals corporation. The fda approved kymriah after a study of 63 patients that found 83% who took the medication us regulators have approved the first cancer drug that uses a patient's own cells to fight cancer. See full prescribing information for kymriah. Date rems call center operational c. Kymriah is a medicine for treating two types of blood cancer: Kymriah™ (tisagenlecleucel) suspension for intravenous do not administer kymriah to patients with active infection or inflammatory disorders. Kymriah faq with patrick brown, m.d. Novartis will continue to build a global. The kymriah approval is a transformational milestone for patients in europe in need of new treatment options, said liz barrett, ceo, novartis oncology. Date kymriah rems website went live b. Kymriah's original approval was for patients under 25 years of age who have relapsed or refractory as ep vantage's jacob plieth points out, the fda calculated kymriah (tisagenlecleucel)'s efficacy.
Regulatory Deep Dive On The Celgene Cvr Nyse Bmy Seeking Alpha - It's Only Fair To Share… Fda Approval Brings First Gene Therapy To The United States 08/30/2017 The U.s.
Frontiers Development And Clinical Translation Of Approved Gene Therapy Products For Genetic Disorders Genetics. Kymriah is a medicine for treating two types of blood cancer: Kymriah™ (tisagenlecleucel) suspension for intravenous do not administer kymriah to patients with active infection or inflammatory disorders. Kymriah faq with patrick brown, m.d. Date hospitals were able to complete rems certification. Fda approval history for kymriah (tisagenlecleucel) used to treat acute lymphoblastic leukemia; The kymriah approval is a transformational milestone for patients in europe in need of new treatment options, said liz barrett, ceo, novartis oncology. The fda approved kymriah after a study of 63 patients that found 83% who took the medication us regulators have approved the first cancer drug that uses a patient's own cells to fight cancer. 15:40 johns hopkins medicine 3 014 просмотров. Date rems call center operational c. Date kymriah rems website went live b. Kymriah's original approval was for patients under 25 years of age who have relapsed or refractory as ep vantage's jacob plieth points out, the fda calculated kymriah (tisagenlecleucel)'s efficacy. Kymriah uses a patient's own immune cells and reengineers them to fight cancer. Novartis will continue to build a global. See full prescribing information for kymriah. Supplied by novartis pharmaceuticals corporation.
Why The Fda Approved Kymriah A Car T Cell Therapy To Treat Cancer Business Insider - Novartis Will Continue To Build A Global.
The Car T Cell Story Open Access Healthbook. Kymriah's original approval was for patients under 25 years of age who have relapsed or refractory as ep vantage's jacob plieth points out, the fda calculated kymriah (tisagenlecleucel)'s efficacy. Kymriah faq with patrick brown, m.d. Novartis will continue to build a global. The fda approved kymriah after a study of 63 patients that found 83% who took the medication us regulators have approved the first cancer drug that uses a patient's own cells to fight cancer. Fda approval history for kymriah (tisagenlecleucel) used to treat acute lymphoblastic leukemia; Date hospitals were able to complete rems certification. See full prescribing information for kymriah. Supplied by novartis pharmaceuticals corporation. Kymriah uses a patient's own immune cells and reengineers them to fight cancer. Kymriah™ (tisagenlecleucel) suspension for intravenous do not administer kymriah to patients with active infection or inflammatory disorders. Kymriah is a medicine for treating two types of blood cancer: 15:40 johns hopkins medicine 3 014 просмотров. Date rems call center operational c. The kymriah approval is a transformational milestone for patients in europe in need of new treatment options, said liz barrett, ceo, novartis oncology. Date kymriah rems website went live b.